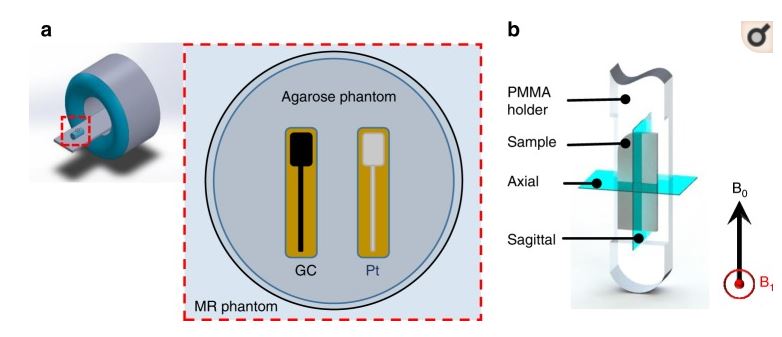
Surabhi Nimalkar, Erwin Fuhrer, Pedro Silva, Tri Nguyen, Martin Sereno, Sam Kassegne and Jan Korvink
Microsystems and Nanoengineering, 2019
The recent introduction of glassy carbon (GC) microstructures supported on flexible polymeric substrates has motivated the adoption of GC in a variety of implantable and wearable devices. Neural probes such as electrocorticography and penetrating shanks with GC microelectrode arrays used for neural signal recording and electrical stimulation are among the first beneficiaries of this technology. With the expected proliferation of these neural probes and potential clinical adoption, the magnetic resonance imaging (MRI) compatibility of GC microstructures needs to be established to help validate this potential in clinical settings. Here, we present GC microelectrodes and microstructures—fabricated through the carbon micro-electro-mechanical systems process and supported on flexible polymeric substrates—and carry out experimental measurements of induced vibrations, eddy currents, and artifacts. Through induced vibration, induced voltage, and MRI experiments and finite element modeling, we compared the performances of these GC microelectrodes against those of conventional thin-film platinum (Pt) microelectrodes and established that GC microelectrodes demonstrate superior magnetic resonance compatibility over standard metal thin-film microelectrodes. Specifically, we demonstrated that GC microelectrodes experienced no considerable vibration deflection amplitudes and minimal induced currents, while Pt microelectrodes had significantly larger currents. We also showed that because of their low magnetic susceptibility and lower conductivity, the GC microelectrodes caused almost no susceptibility shift artifacts and no eddy-current-induced artifacts compared to Pt microelectrodes. Taken together, the experimental, theoretical, and finite element modeling establish that GC microelectrodes exhibit significant MRI compatibility, hence demonstrating clear clinical advantages over current conventional thin-film materials, further opening avenues for wider adoption of GC microelectrodes in chronic clinical applications.